NEED TO TURN DOWN IOP?
TURN TO THE STRENGTH OF
SIMBRINZA® SUSPENSION1-4

SIMBRINZA® Suspension has clinically proven efficacy as both adjunct and first-line therapies in patients with open-angle glaucoma or ocular hypertension.2-4
Indications
SIMBRINZA® (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is a fixed combination indicated in the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Contraindications
SIMBRINZA® is contraindicated in patients who are hypersensitive to
any component of this product and neonates and infants under the
age of 2 years.
References: 1. SIMBRINZA® Suspension [prescribing information]. Fort Worth, TX: Alcon Laboratories Inc; 2015. 2. Fechtner RD, Myers JS, Hubatsch DA, Budenz DL, DuBiner HB. Ocular hypotensive effect of fixed-combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial [published online July 1, 2016]. Eye (Lond). 2016;30(10):1343-1350. doi:10.1038/eye.2016.126. 3. Katz G, DuBiner H, Samples J, Void S, Sall K. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013;131(6):724-730. 4. Nguyen QH, McMenemy MG, Realini T, Whitson JT, Goode SM. Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%. J Ocul Pharmacol Ther. 2013;29(3):290-297.
Efficacy: Adjunct to PGA
SIMBRINZA® Suspension reduced IOP when added to a PGA1
SIMBRINZA® Suspension should be taken at least five (5) minutes apart from other topical ophthalmic drugs.2
Delivered up to 7.1 mm Hg additional IOP reduction from baseline when added to a PGA1

Primary End Point1
Mean diurnal IOP (IOP averaged over all daily time points) at week 6 between treatment groups
Key Secondary End Points1
Mean IOP for each daily time point at week 6
Mean diurnal change from baseline to week 6
*Differences (mm Hg) at week 6 time points between treatment groups were -2.1 (P=0.0002), -4.6 (P<0.0001), -2.8 (P<0.0001), and -4.4 (P<0.0001)1

5.7 mm Hg additional mean diurnal IOP reduction from baseline at week 6 when added to a PGA1

STUDY DESIGN1
A prospective, randomized, multicenter, double-blind, parallel-group study of 182 patients with open-angle glaucoma and/or ocular hypertension receiving treatment with a PGA. PGA treatment consisted of travoprost, latanoprost, or bimatoprost. Patients in the study were randomized to adjunctive treatment with SIMBRINZA® Suspension (n=88) or vehicle (n=94). The primary efficacy end point was mean diurnal IOP (IOP averaged over all daily time points) at week 6 between treatment groups. Key secondary end points included mean IOP at week 6 for each daily time point (8 AM, 10 AM, 3 PM, and 5 PM) and mean diurnal IOP change from baseline to week 6.
References: 1. Fechtner RD, Myers JS, Hubatsch DA, Budenz DL, DuBiner HB. Ocular hypotensive effect of fixed-combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial [published online July 1, 2016]. Eye (Lond). 2016;30(10):1343-1350. doi:10.1038/eye.2016.126. 2. SIMBRINZA® Suspension [prescribing information]. Fort Worth, TX: Alcon Laboratories Inc; 2015.
Efficacy: Monotherapy
SIMBRINZA® Suspension reduced IOP in clinical trials as monotherapy1,2


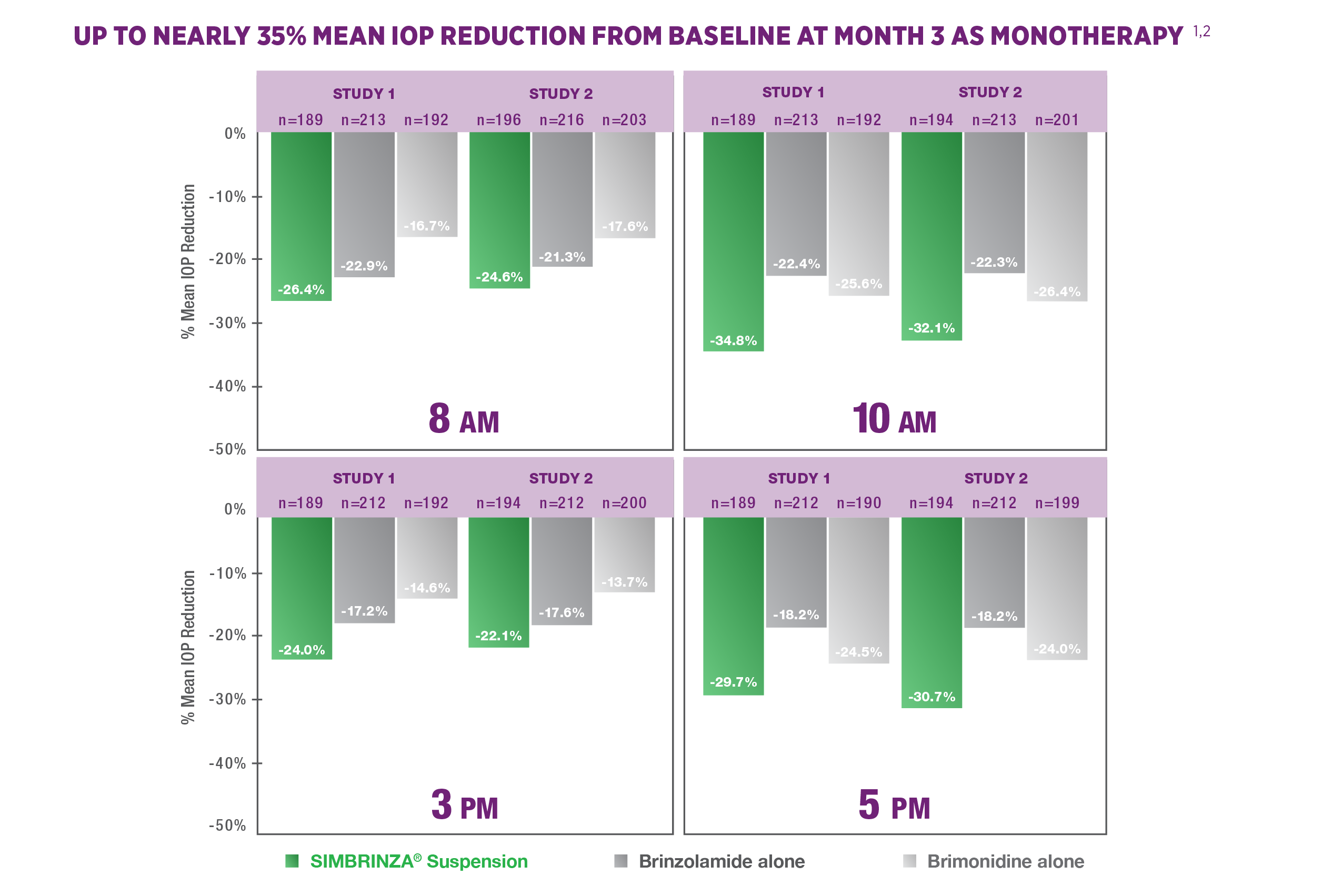
STUDY DESIGN1-3
Intent-to-treat data from two 3-month clinical studies (N=649; N=679). Patients with ocular hypertension or open-angle glaucoma with elevated IOP were given SIMBRINZA® Suspension 3 times a day (tid); brinzolamide ophthalmic suspension, 1%, 3 times a day (tid); or brimonidine tartrate ophthalmic solution, 0.2%, 3 times a day (tid). Patients’ mean IOPs at baseline were 21 to 36 mm Hg. Dosing times were at 8 AM, 3 PM, and 10 PM. For study 1 at baseline: SIMBRINZA® Suspension, n=209; brinzolamide, n=224; brimonidine, n=216. For study 2 at baseline: SIMBRINZA® Suspension, n=218; brinzolamide, n=229; brimonidine, n=232. Study 1 compared the IOP-lowering efficacy of SIMBRINZA® Suspension with the efficacy of its components brinzolamide and brimonidine. Primary end point for study 1 was the mean IOP at the 3-month visit at all time points: 8 AM, 10 AM, 3 PM, and 5 PM. Study 2 assessed the superiority of the IOP-lowering efficacy of SIMBRINZA® Suspension vs brinzolamide alone and brimonidine alone. Primary end point for study 2 was the mean IOP at 3 months at all time points: 8 AM, 10 AM, 3 PM, and 5 PM.
References: 1. Katz G, DuBiner H, Samples J, Void S, Sall K. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013;131(6):724-730. doi:10.1001/jamaophthalmol.2013.188. 2. Nguyen QH, McMenemy MG, Realini T, Whitson JT, Goode SM. Phase 3 randomized 3-month trial with an ongoing 3-month safety extension of fixed combination brinzolamide 1%/brimonidine 0.2%. J Ocul Pharmacol Ther. 2013;29(3):290-297. 3. SIMBRINZA® Suspension [prescribing information]. Fort Worth, TX: Alcon Laboratories Inc; 2015.
Who is an appropriate patient?
See how SIMBRINZA® Suspension may help your patients
When additional IOP reduction may be needed for a patient on PGA therapy
SIMBRINZA® Suspension should be taken at least five (5) minutes apart from other topical ophthalmic drugs.1
Ann, 77*
Patient Details
- Open-angle glaucoma patient with elevated IOP
- On PGA therapy that has reduced her IOP from 26 mm Hg OU to 18 mm Hg OU for several years
- Maintains an active lifestyle with an upbeat, independent attitude
Status
- Recently, Ann's IOP has risen to around 22 mm Hg OU, and optic nerve damage has progressed
- She is cognizant of the need to work toward a target IOP and a reduction of approximately 5 mm Hg
Treatment Plan
Consider adding SIMBRINZA® Suspension to a PGA


When you want to replace a single component with a combination
Joe, 67*
Patient Details
- Open-angle glaucoma patient
- Currently treated with a PGA and brimonidine 0.2%
- No comorbid conditions
Status
- Joe was started on a prostaglandin 6 months ago and achieved IOP lowering
- He was later prescribed brimonidine 0.2% and obtained additional IOP control, but he still needs additional IOP reduction to reach his target IOP
- He is concerned about adding another bottle to his regimen
Treatment Plan
- Consider using SIMBRINZA® Suspension to replace Joe's brimonidine 0.2% to maintain his burden of therapy (number of bottles used per day) while offering additional IOP lowering
- Counsel Joe about lowering his IOP and prescribe him SIMBRINZA® Suspension

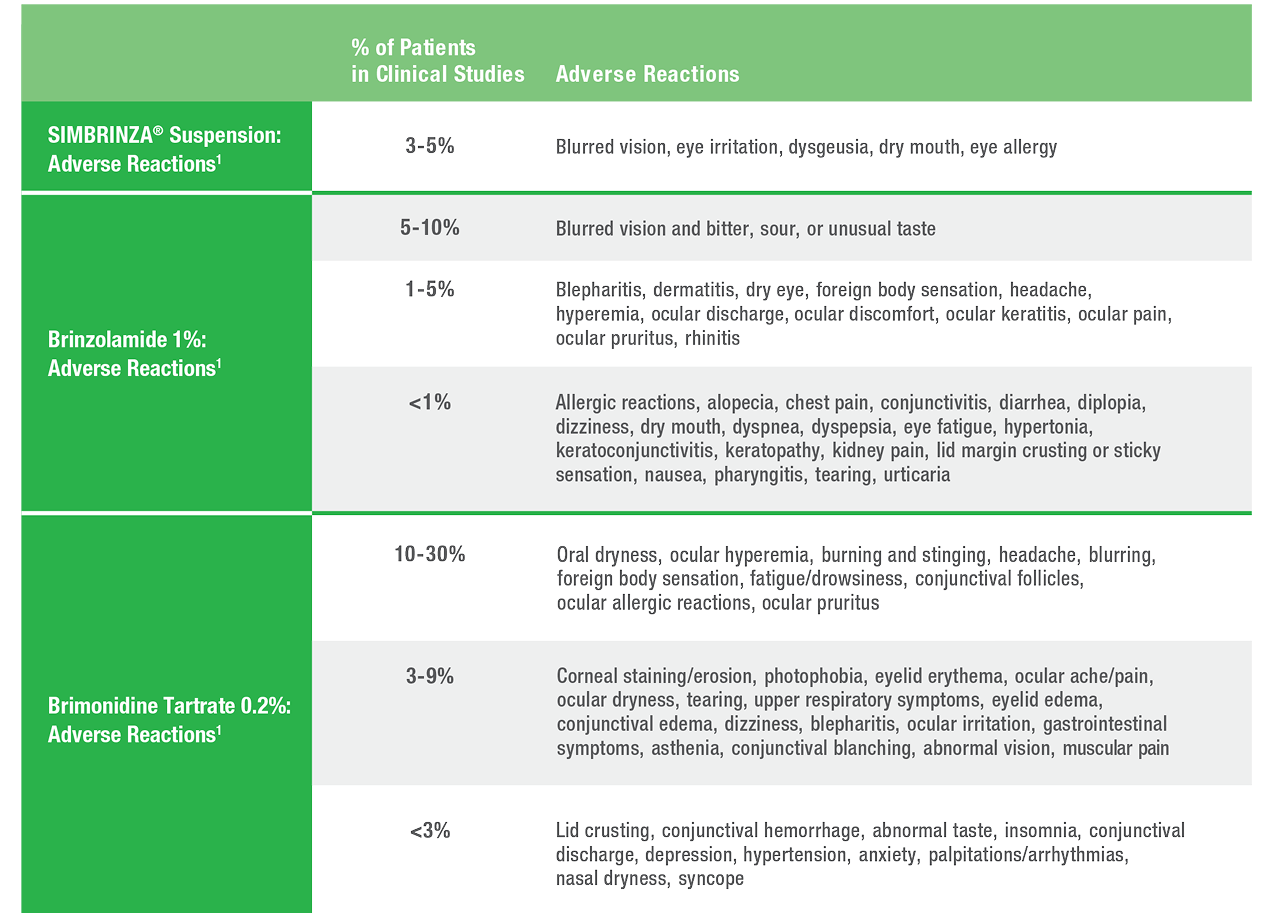
Safety
SIMBRINZA® Suspension Adverse Reactions
Reference: 1. SIMBRINZA® Suspension [prescribing information]. Fort Worth, TX: Alcon Laboratories Inc; 2015.
At Alcon, we know patient access to medications is important.
We’ve created a prescription co-pay savings program that's simple to use and can help eligible patients with out-of-pocket costs.
IMPORTANT SAFETY INFORMATION
Contraindications
SIMBRINZA® is contraindicated in patients who are hypersensitive to any component of this product and neonates and infants under the age of 2 years.
INDICATIONS AND USAGE
SIMBRINZA® (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is a fixed combination indicated in the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Warnings and precautions
Sulfonamide Hypersensitivity Reactions—Brinzolamide is a sulfonamide, and although administered topically, is absorbed systemically. Sulfonamide attributable adverse reactions may occur. Fatalities have occurred due to severe reactions to sulfonamides. Sensitization may recur when a sulfonamide is readministered irrespective of the route of administration. If signs of serious reactions or hypersensitivity occur, discontinue the use of this preparation immediately.
Corneal Endothelium—There is an increased potential for developing corneal edema in patients with low endothelial cell counts. Caution should be used when prescribing SIMBRINZA to these patients.
Severe Renal Impairment (CrCl <30 mL/min)—SIMBRINZA has not been specifically studied and since brinzolamide and its metabolite are excreted primarily by the kidney, is not recommended in these patients.
Acute Angle-Closure Glaucoma—The management of patients with acute angle-closure glaucoma requires therapeutic interventions in addition to ocular hypotensive agents. SIMBRINZA has not been studied in these patients.
Contact Lens Wear—The preservative in SIMBRINZA, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of SIMBRINZA but may be reinserted 15 minutes after instillation.
Severe Cardiovascular Disease—Brimonidine tartrate, a component of SIMBRINZA, had a less than 5% mean decrease in blood pressure 2 hours after dosing in clinical studies; caution should be exercised in treating patients with severe cardiovascular disease.
Potentiation of Vascular Insufficiency—Brimonidine tartrate, a component of SIMBRINZA, may potentiate syndromes associated with vascular insufficiency. It should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud’s phenomenon, orthostatic hypotension, or thromboangiitis obliterans.
Contamination of Topical Ophthalmic Products After Use—There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers have been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
Adverse Reactions
SIMBRINZA® Suspension
In two clinical trials of 3 months’ duration with SIMBRINZA, the most frequent reactions associated with its use occurring in approximately 3-5% of patients in descending order of incidence included: blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, and eye allergy. Adverse reaction rates with SIMBRINZA were comparable to those of the individual components. Treatment discontinuation, mainly due to adverse reactions, was reported in 11% of SIMBRINZA patients.
Brinzolamide 1%
In clinical studies of brinzolamide ophthalmic suspension 1%, the most frequently reported adverse events reported in 5-10% of patients were blurred vision and bitter, sour, or unusual taste.
Brimonidine Tartrate 0.2%
In clinical studies of brimonidine tartrate 0.2%, adverse events occurring in approximately 10-30% of the subjects, in descending order of incidence, included oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus.
Drug Interactions
Concomitant administration with oral carbonic anhydrase inhibitors is not recommended due to the potential additive effect. Use with high-dose salicylate may result in acid-base and electrolyte alterations. The possibility of an additive or potentiating effect with CNS depressants should be considered. Because brimonidine tartrate may reduce blood pressure, caution in using antihypertensives and/or cardiac glycosides with SIMBRINZA is advised. Use of tricyclic antidepressants may blunt the hypotensive effect of systemic clonidine and it is unknown if use of this class of drugs with SIMBRINZA interferes with IOP lowering. Use with monoamine oxidase inhibitors may result in increased systemic side effects, such as hypotension.